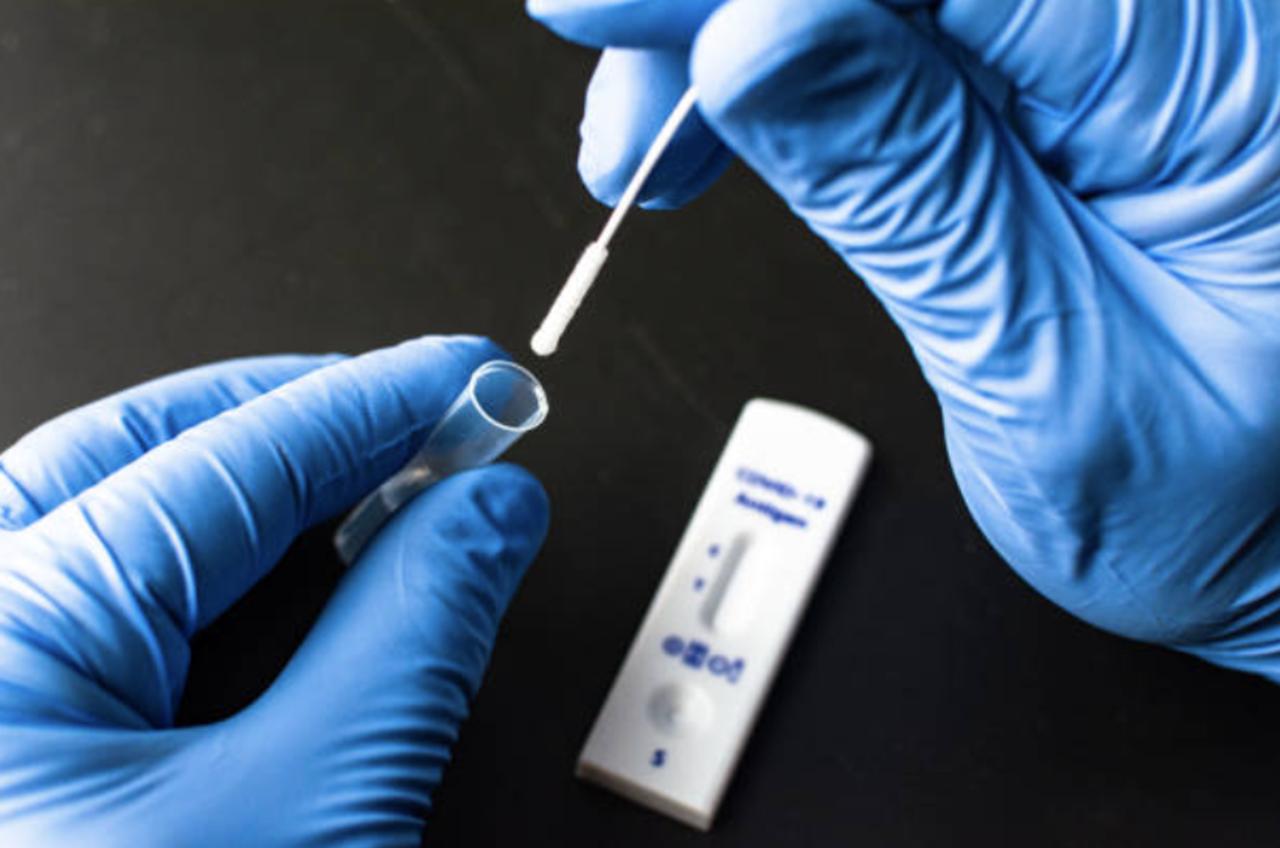
2nd At-Home Rapid COVID Test , Is Authorized by the FDA.
The U.S. Food and Drug Administration (FDA) announced its authorization on Dec.
28.
The test was designed by Siemens Healthineers and can now be used in addition to the Roche test the FDA also recently approved.
The authorization comes as test shortages have wracked the nation amid record-breaking numbers of infections.
Officials with the Biden administration praised the FDA's move as in keeping with its COVID priorities.
Increasing Americans' access to easy-to-use, reliable COVID tests is a top priority for the Biden Administration, , Xavier Becerra, Health and Human Services Secretary, via 'The Hill'.
... and we are using all resources at our disposal to make more tests available and ramp up supply, Xavier Becerra, Health and Human Services Secretary, via 'The Hill'.
With daily infections topping 300,000 in the U.S., at-home testing has become a signature administration strategy.
Adding two new authorized tests will give Americans more options for testing at home, , Xavier Becerra, Health and Human Services Secretary, via 'The Hill'.
... which helps keep people safe and provides peace of mind, Xavier Becerra, Health and Human Services Secretary, via 'The Hill'.
FDA officials cautioned that the at-home test is not 100 percent accurate.
Negative results do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions.
, Jacqueline A.
O’Shaughnessy, FDA Scientist, via 'The Hill'.
Despite this, the tests offer reliable guidance in the context of a person's potential exposure to COVID-19.
Negative results should be considered in the context of an individual’s recent exposures, history and the presence of clinical signs and symptoms consistent with COVID-19, Jacqueline A.
O’Shaughnessy, FDA Scientist, via 'The Hill'.
The Biden administration is expected to begin distribution of 500 million at-home tests sometime in January
