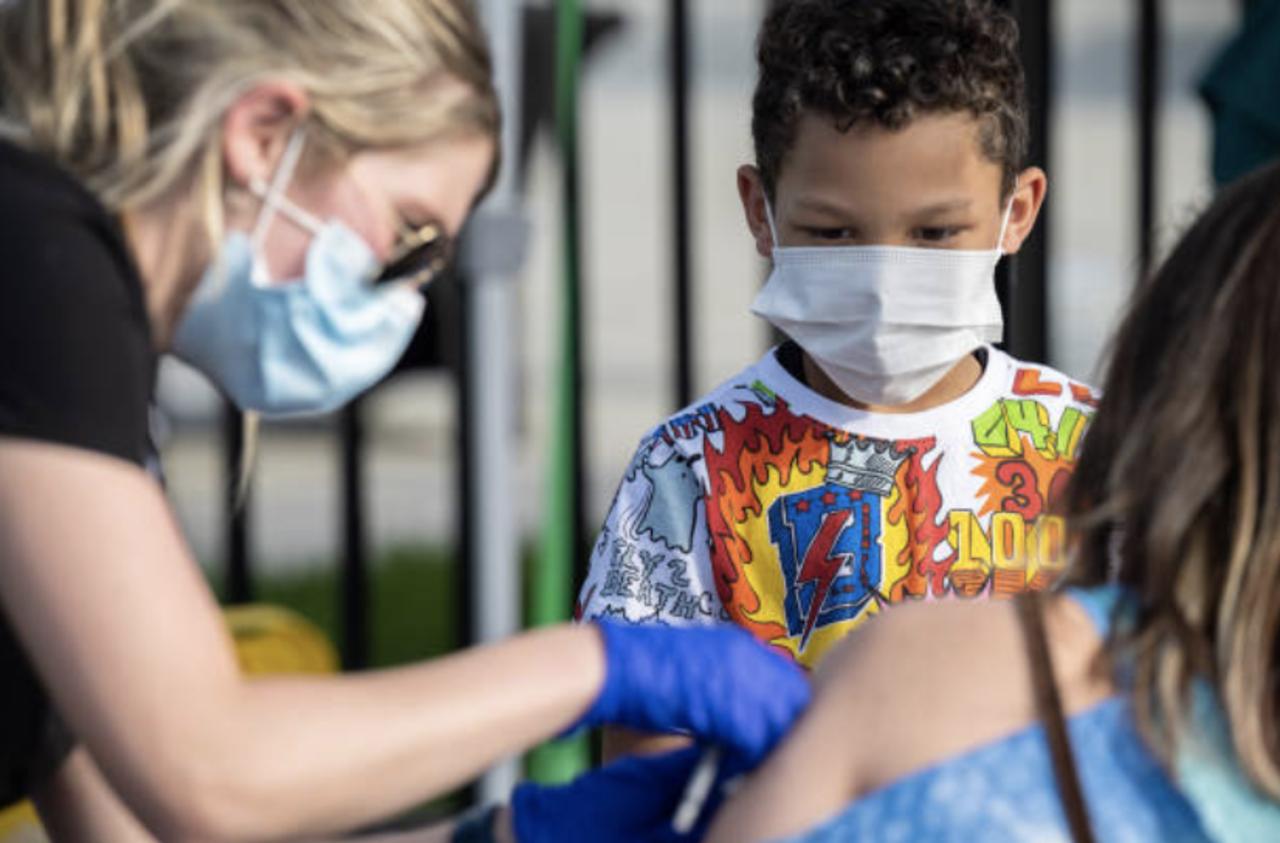
Pfizer Seeks FDA Authorization , To Use COVID Vaccine for Children Ages 5 to 11.
Pfizer and BioNTech announced they were seeking approval from the U.S. Food and Drug Administration (FDA) on Oct.
7.
In the U.S., the Pfizer COVID-19 vaccine has been approved for those who are 16 and older.
In the EU, children ages 12 through 15 are able to receive the vaccine.
Pfizer released data last month from a Phase 2/3 trial which showed that its COVID-19 vaccine was safe... .
... and provided a "robust" antibody response to the virus in children between the ages of five and 11.
The dosage used for children between these ages was a third of the dosage used in people 15 and older.
Now that Pfizer has officially requested approval for use of the vaccine in children... .
... FDA officials have indicated that with supporting data, it could be approved in a matter of weeks.
The FDA Vaccines and Related Biological Products Advisory Committee is set to meet to discuss authorization on Oct.
26.
The acting director of the FDA recently outlined the considerations for the upcoming committee meeting.
We know from our vast experience with other pediatric vaccines that children are not small adults, .., Dr. Janet Woodcock, Acting FDA Commissioner, via CNN.
... and we will conduct a comprehensive evaluation of clinical trial data submitted in support of the safety and effectiveness of the vaccine used in a younger pediatric population, .., Dr. Janet Woodcock, Acting FDA Commissioner, via CNN.
... which may need a different dosage or formulation from that used in an older pediatric population or adults, Dr. Janet Woodcock, Acting FDA Commissioner, via CNN.
If approved by the FDA, a Centers for Disease Control and Prevention (CDC) committee will then consider approval as well










